In vivo Measurement of Knee Extensor Muscle Function in Mice
Knee extensor muscul[...]
Disparate bone anabolic cues activate bone formation by regulating the rapid lysosomal degradation of sclerostin protein
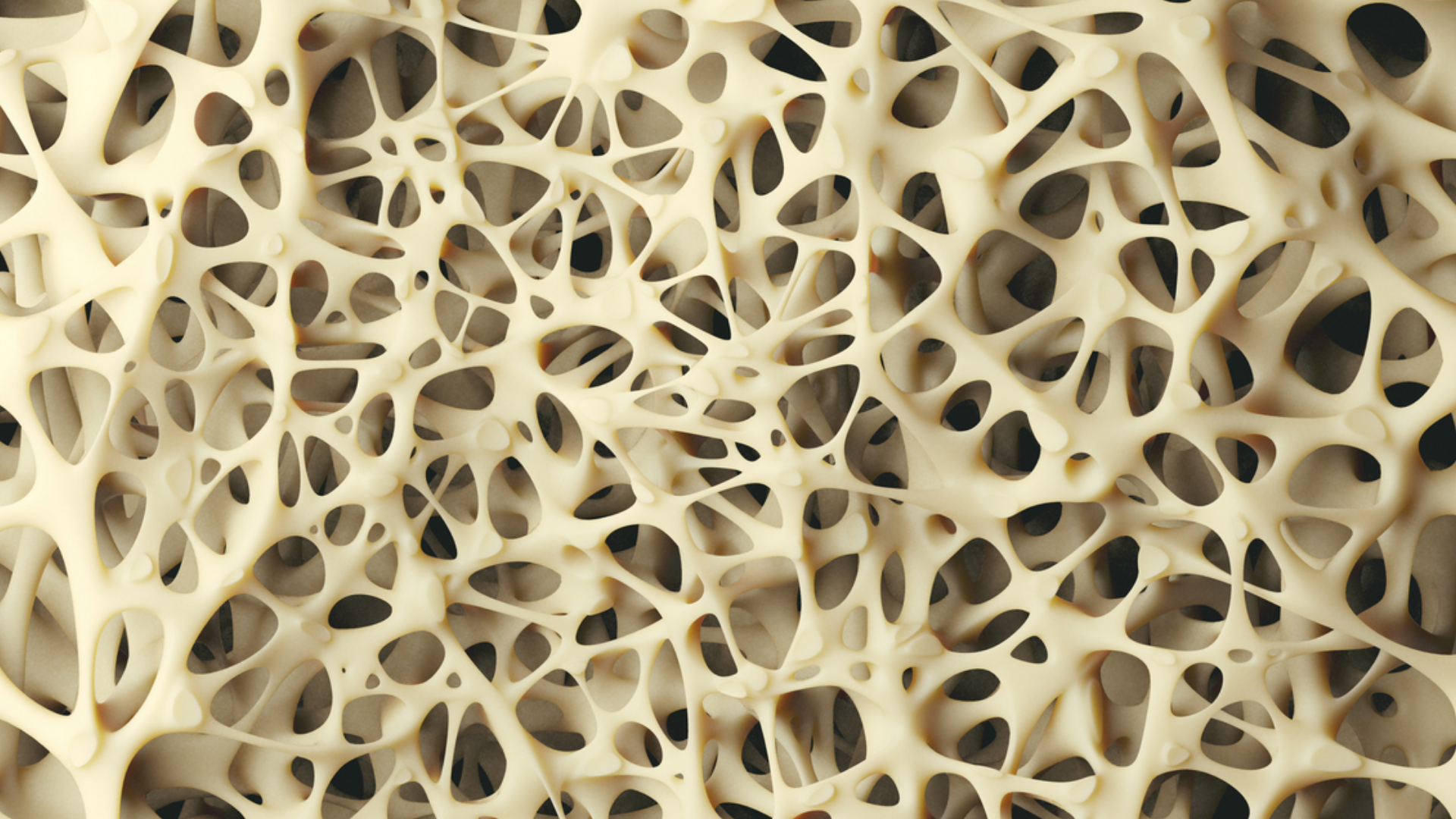
Sclerostin is an osteocyte-derived glycoprotein with an inhibitory role in bone formation. Deletion of the Sost gene, which controls sclerostin protein expression, has been shown to increase bone mass in mice. As such, sclerostin shows therapeutic potential in treating bone mass conditions such as osteoporosis. This study focuses on elucidating the molecular control of this protein in both in vitro and in vivo settings. Cell lines Ocy454 and UMR106 were first subjected to fluid shear stress-mediated bone mechanical loading. This resulted in a loss of sclerostin protein abundance. An in vivo assessment was then conducted where mice were subjected to ulnar loads using Aurora’s 305C Dual-Mode lever system. Ulnae were harvested post-load and osteocytes were assessed, showing reduced sclerostin abundance compared to limbs without loading. A previous study showed that NOX2-mediated ROS is an essential part of the mechano-transduction pathway involving sclerostin protein loss. To confirm these findings in a whole animal analysis, the authors analyzed the effect of ulnar loading on mice pre-treated with apocynin, a NOX2 inhibitor. The findings showed that apocynin prevented bone formation that was otherwise load-induced. Therefore, NOX2 ROS was found to be necessary for sclerostin degradation as inhibition of NOX2 lessened the degradation of sclerostin. Furthermore, it was found that inhibition of lysosomal degradation reduced sclerostin protein degradation. These results suggest that inhibiting NOX2 or lysosomal function prevents load-induced sclerostin degradation and subsequent bone formation. The authors then analyzed induced pluripotent stem cell (iPSC)-derived osteoblasts from patients with Gaucher disease, a disease categorized as a lysosomal storage disorder. This analysis showed a significant increase in levels of sclerostin compared to iPSC-derived osteoblasts from patients without Gaucher disease. It was also found that treating Gaucher iPSC-derived osteoblasts with an enzyme restoring lysosomal function resulted in a decrease in sclerostin. These data provide insights into the molecular regulation of osteocyte sclerostin protein and shed light on potential therapeutic targets in the treatment of bone-related conditions.
A Step Further: Customizing the 3-in-1 Whole Animal System
In scientific exper[...]
Tips and Tricks for Troubleshooting the 300C Dual-Mode Muscle Levers
Here we will cover [...]
Tips and Tricks for Measuring Muscle Function in-situ
Aurora Scientific’s[...]
A Look at Bone-Muscle Crosstalk From in-vivo and in-vitro Perspectives
The bone-muscle int[...]
Tips and Tricks for Measuring Muscle Function in-vitro
Aurora Scientific’s[...]
Tips and Tricks for Measuring Muscle Function in-vivo
Aurora Scientific’s[...]
Commonly Used Samples and Experiments using Whole Animal Systems
Aurora Scientific o[...]
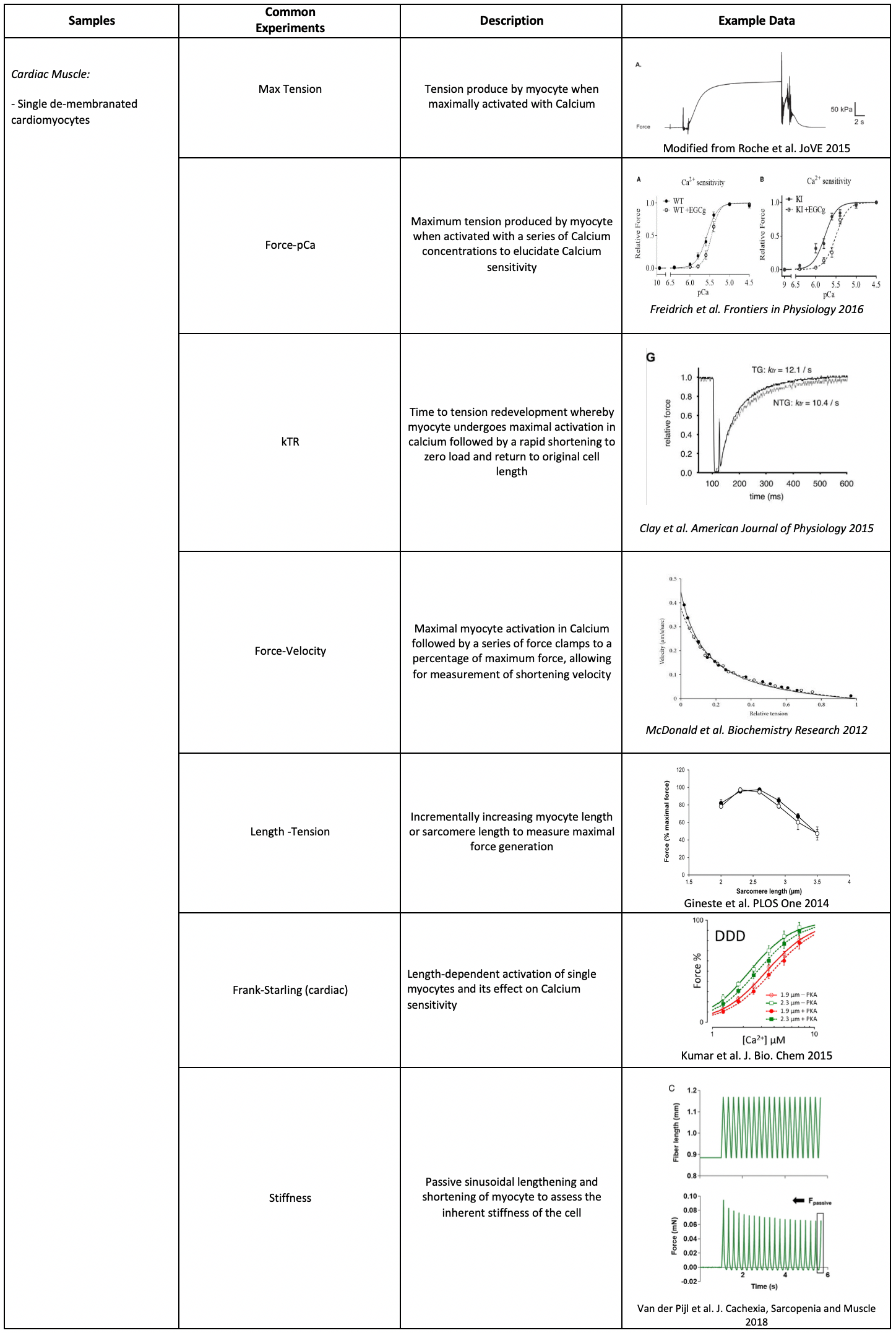
Commonly Used Samples and Experiments for Aurora Scientific Systems
Aurora Scientific of[...]
Experimental Methods for In-Vivo (Footplate) Procedures
This blog is intende[...]
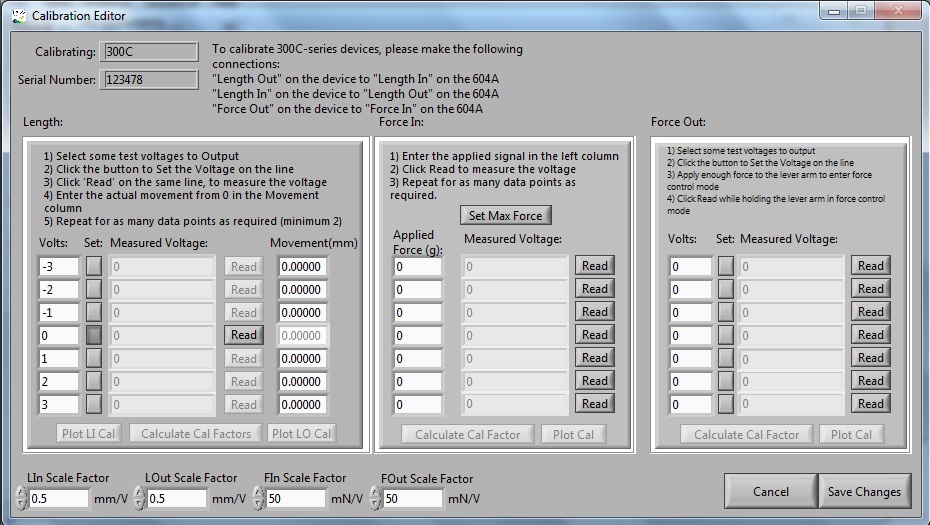
How to Calibrate Your Dual-Mode Lever System Using DMC
This blog is intende[...]
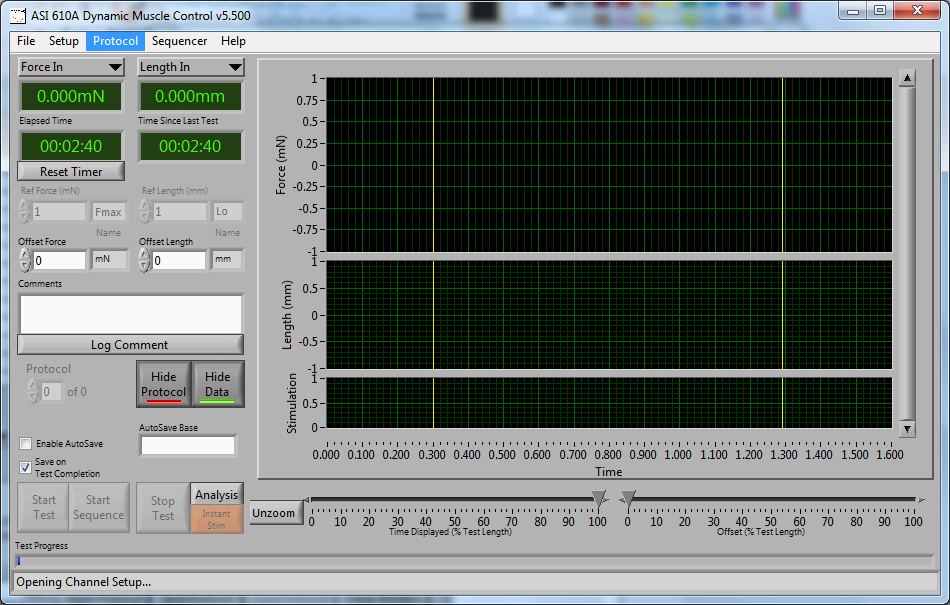
How to Write Basic Protocols in DMC – Isotonic
Are you wondering h[...]
How to Write Basic Protocols in DMC – Concentric and Eccentric
Are you looking to [...]
Beyond a Force Transducer: 300C Capabilities
Are you familiar wit[...]
How to Write Basic Protocols in DMC – Isometric
Looking to character[...]
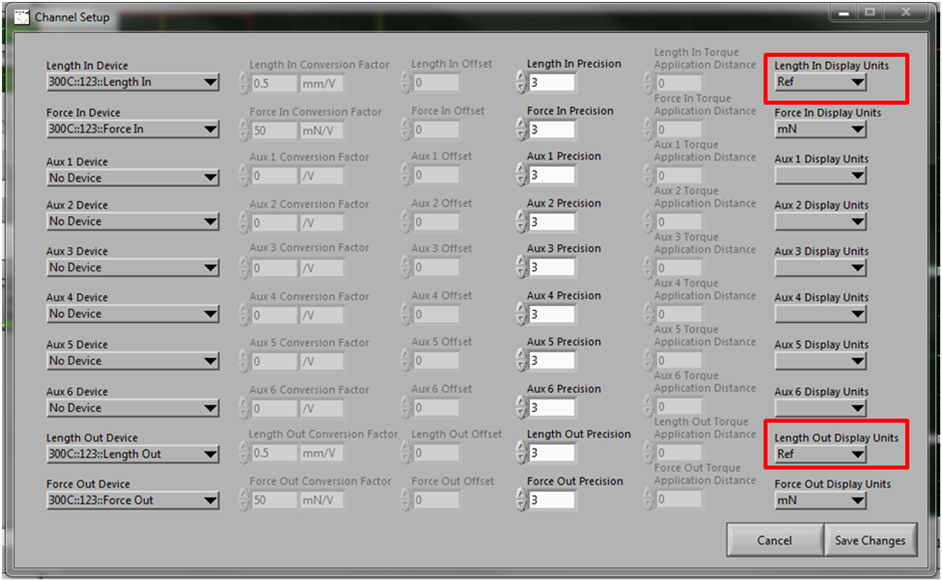
Control Muscle Length with DMC Software Using Reference Units
One of the most use[...]
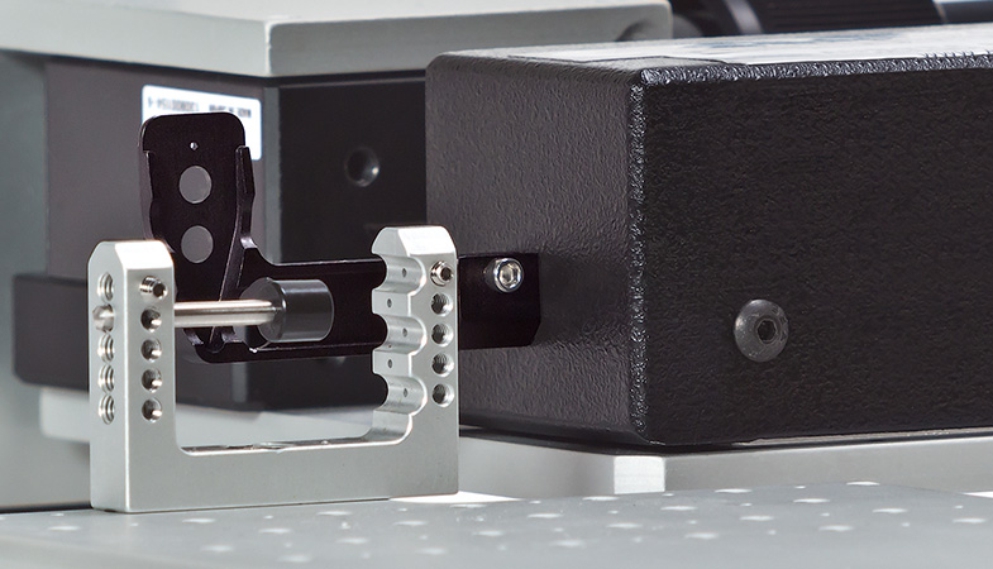
Necessary Equipment to Operate Your New ASI Muscle Test System
You've received a s[...]